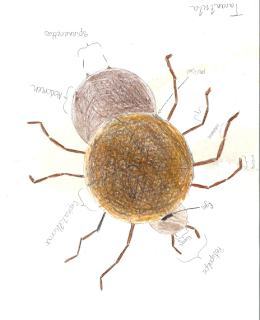
Saturday, October 5, 2013
Sunday, September 15, 2013
Stoichimetry f a Precipitation Reaction
Questions
A. From your
balanced equation what is the theoretical yield of your product?
I got
B.
According to your data table, what is the
actual yield of the product?
64.6g
64.6g
C. A perfect
percent yield would be 100%. Based on your results, comment on your degree of
accuracy and suggest possible sources of error.
Possible sources of error could be occurring in the weigh boat.
D. How could
these errors be reduced in the future?
Use the water to make the weigh boat clean and thereby getting all into the mixture.
Sunday, September 8, 2013
Substance: Mg
Color: red-brown
Odor: metallic
Effect of Heat: crunched
Litmus test: solid
Substance: Cu
Color: reddish
brown
Odor: metallic
Effect of Heat: holes
developed
Litmus test: purple
Substance: Zn
Color: silvery
Odor: metallic
Effect of Heat: develops
holes and steams
Litmus test: purple
Substance: NaCl
Color: opaque white
Odor: salty
QUESTIONS:
A - Did you observer any
chemical changes in this experiment?
yes
B - What evidence
did you use to decide that something was a chemical change?
element and reactions
element and reactions
C -
Give at least two examples of chemical changes you observed.
steaming, formations
D -
Classify the following properties of sodium metal as physical or chemical:
Silver metallic color – physical
Turns gray in air – chemical
Melts at 98oC - physical
Reacts explosively with chlorine - chemical
E - Classify the following changes as physical or chemical:
Water freezes at OoC
- physical
Baking soda when
combined with vinegar produces bubbles:- chemical
Mothballs gradually
disappear at room temperature – physical
Ice cubes in a freezer
get smaller with time – physical
Baking soda loses mass
as it is heated – chemical
Tarnishing of silver – physical
F - How would you show that dissolving table salt is a physical change?
Dehydrate the solution
Tuesday, August 20, 2013
Fermentation of Ginger Ale
In the past few weeks,
we've made ginger ale.
Start by making a ginger
bug:
Ingedients: 1 fresh
ginger root, 1/2 cup white granulated sugar, two cups of water, and a quart
sized mason jar
Instructions:
1) Slice a 1.5 inch long
ginger root piece and peel the root grate it to make grated ginger.
2) Put the grated ginger
in the mason jar and add sugar in the equivalent amount.
3) Add the two cups of
water.
4) Put a breathable lid
on the jar.
5) Daily for five days,
add a tablespoon of both the sugar and the grated ginger.
6) After adding, stir
it in with a nonmetal spoon, and cover.
7) The culture can be
verified active if bubbles form at the top and it fizzes.
8) Do not allow the
culture to cross contaminate.
Now start the actual
ginger ale part:
Ingredients: 2 in. piece
of fresh minced ginger root, ½ cup sugar, 1 tbs molasses,1/2 cup lemon juice, 8
cups of filtered water, ½ tsp of sea salt.
Instructions:
1) Make a ginger wort by
boiling 3 cups of water, the minced ginger root, sugar, molasses, and salt in a
saucepan.
2) Let the mixture
simmer for five or so minutes as long as the sugar is entirely dissolved.
3) Remove the mixture
from heat and add the water, then allow it to cool to room temperature.
4) Add lemon juice to
the mixture.
5) Place the mixture in
the 2 quart jar and tightly place the lid after stirring the mixture.
6) Set it on the counter
for two or three days for the carbonation and fermentation to set it.
7) Strain the mixture
before drinking it.
Our Experience:
The ginger can become
overbearing but is better when refrigerated. This come out to be more flavorful
than store-bought.
Credit to: Wellness Mama
for the recipes.
Sunday, August 11, 2013
Properties of Gases
Questions
A. Give two reasons why we fill the gas
generator test tubes almost to the top with chemicals.
B. What happens to the zinc in the
hydrogen generation experiment?
Darkening of metal
C. What happens to the manganese in the
oxygen generation experiment?
Melding
of separate bits
D. Write a balanced equation for the
reaction between O2 and H2.
O2+2H2=H2O
E. What is the function/purpose of the
bromthymol blue in the CO2 experiment?
The
color changes in presence of solutions
F.
Bromothymol
blue is blue in the presence of basic solutions, and yellow in the presence of
acidic solutions. If your solution is a murky green, what might you assume
about the solution?
Neutral
G. Make a table of the gases studied.
Tabulate their colors, the effect on lighted or glowing splints, and other
properties one might use to identify them.
Sample table:
Gas
|
Flame reaction
|
Glowing splint
|
Limewater reaction
|
Bromthymol blue
react.
|
Hydrogen
|
NA
|
NA
|
NA
|
|
Oxygen
|
NA
|
NA
|
||
Hydrogen & oxygen
|
NA
|
NA
|
NA
|
|
Carbon dioxide
|
NA
|
|||
Alka Seltzer
|
NA
|
|||
Breath
|
NA
|
Sunday, July 7, 2013
Measurements Lab
Length measurements
|
Object
|
Object Length (cm)
|
Length (mm)
|
|
Science CD
|
12
|
120
|
|
Laptop Trackpad
|
9
|
90
|
|
Alexia’s Invitation
|
14
|
140
|
Temperature measurements
|
Hot water from tap (ºC )
|
Boiling water (ºC)
|
Boiling water-5 minutes(ºC)
|
|
37.78
|
|
|
|
Cold water from tap (ºC)
|
Ice water (ºC)
|
Ice water-5 minutes (ºC)
|
|
|
|
|
Could not complete the tests because there was an issue with our items.
Volume Measurements
|
Test tube volume (mL)
|
Number of drops in 1 mL
|
Pipet volume (mL)
|
|
11mL
|
3
|
3
|
Mass Measurements
|
Object
|
Estimated Mass (g)
|
Actual Mass (g)
|
|
Stirring Stick
|
15
|
11.1
|
|
Favourite Pencil
|
10
|
8.3
|
|
End Roll of Cast Tape
|
5
|
6.7
|
|
Metal Burn Plate
|
35
|
42.2
|
|
Wireless Mouse
|
100
|
93.9
|
|
Silver Nitrate Bottle
|
5
|
7.1
|
|
Mobile Phone
|
125
|
91.5
|
Density Measurements
|
|
Mass A
|
Mass B
|
A-B
|
|
|
|
Object
|
Cylinder+Substance
|
Cylinder
|
Substance
|
Volume
|
Density(M/V)
|
|
Water
|
21.4g
|
16.6g
|
4.8g
|
5mL
|
0.96g/mL
|
|
Rubbing Alcohol
|
21.4g
|
16.6g
|
4.8g
|
5mL
|
0.96g/mL
|
|
Salt Solution
|
22.4g
|
16.6g
|
5.8g
|
5mL
|
1.16g/mL
|
Monday, June 17, 2013
Calorimeter
How many calories does it take to raise the temperature of
one gram of water by one degree?
We will determine calorie content of varying foods using a homemade calorimeter.
We will determine calorie content of varying foods using a homemade calorimeter.
Procedure:
1.
Measure 25 g of each substance.
2.
Measure out 100 g of water.
3.
Grab all materials.
4.
Make chart.
5.
Light food aflame.
6. Record Data.
6. Record Data.
Ocean Creatures
|
25
|
19
|
22
|
3
|
|
Chocolate Cremes
|
24
|
22
|
22
|
-1
|
|
Swirly Gummy Bears
|
25
|
22
|
22
|
||
Potato Chips
|
24
|
22
|
32
|
10
|
-1
|
Sweet Potato Chips
|
13
|
35
|
53
|
18
|
-12
|
Tortilla Chips
|
14
|
33
|
64
|
31
|
-11
|
Sunflower Seeds
|
28
|
27
|
35
|
8
|
+3
|
Pumpkin Seeds
|
24
|
32
|
34
|
2
|
-1
|
Roundy Crackers
|
27
|
34
|
34
|
+2
|
|
Pita Chips
|
21
|
31
|
55
|
24
|
-4
|
Peaches
|
25
|
33
|
|||
Mangos
|
25
|
33
|
|||
Orange Sugar Wafers
|
26
|
33
|
39
|
6
|
+1
|
Nilla Wafers
|
26
|
35
|
|||
Marshmallows
|
23
|
22
|
32
|
10
|
-2
|
Observations:
Ocean Creatures
|
Flame died out a lot
|
Chocolate Cremes
|
Refused to light aflame
|
Swirly Gummy Bears
|
Inflammable
|
Potato Chips
|
Flickered in and out of flame
|
Sweet Potato Chips
|
Crazy good burn
|
Tortilla Chips
|
Flame quickly
|
Sunflower Seeds
|
Died out quickly and consistently
|
Pumpkin Seeds
|
Inflammable
|
Roundy Crackers
|
Char better than they burn
|
Pita Chips
|
Quick and awesome burn
|
Peaches
|
Inflammable
|
Mangos
|
Inflammable
|
Orange Sugar Wafers
|
Edges burnt very well
|
Nilla Wafers
|
Inflammable
|
Marshmallows
|
Crispy and yummy looking
|
Calculations for those that could be done:
Ocean Creatures
|
(100g)(3C)=300g/C
|
Potato Chips
|
(100g)(10C)=1000g/C
|
Sweet Potato Chips
|
(100g)(18C)=1800g/C
|
Tortilla Chips
|
(100g)(31C)=3100g/C
|
Sunflower Seeds
|
(100g)(8C)=800g/C
|
Pumpkin Seeds
|
(100g)(2C)=200g/C
|
Pita Chips
|
(100g)(24C)=2400g/C
|
Marshmallows
|
(100g)(10C)=1000g/C
|
Subscribe to:
Comments (Atom)